Why Is the Formation of a Solution a Spontaneous Process
Melting of ice is a spontaneous process because liquid state is more random than the solid state. A homogeneous solution would form if we waited long enough.

General Chemistry Notes Full Course Pdf Notes Chemistrynotes Com Chemistry Notes General Chemistry Notes Chemistry
1 a decrease in the internal energy of the system an exothermic change - occurs as heat is absorbed or evolvedreleased.

. Indications of spontaneous formation of precursor proteins. Ideal solutions may also form when structurally similar liquids are. When NO₂ dimerizes two molecules join together to form a dimer into N₂O₄ an equilibrium is reached and in.
Explain why the formation of a solution is a spontaneous process. What are some traits of a solution. The formation of a solution is an example of a spontaneous process a process that occurs under specified conditions without the requirement of energy from some external source.
Scientists from the Institute of Organic Chemistry at the University of Stuttgart have determined that under certain conditions spontaneous reactions take place between ribonucleotides and amino acids leading to molecules that contain ribonucleic acids RNA as well as peptides. So thats a fancy term for a spontaneous and action and biology so and energetically favourable is also spontaneous. An iron object rusts in moist air.
Entropydisorder increase reaction starts spontaneously even if endothermic molecules unrestrained spontaneous mixing occurs. Ice turning to water is spontaneous at T 0C Water turning to ice is spontaneous at T 0C. These processes occur without requiring an outside force and continue until equilibrium.
Explain why the formation of a solution is a spontaneous process. A spontaneous process is a process that occurs on its own without outside intervention. Energy decrease reaction starts spontaneously.
Have no effect because adding more solid will have no effect. Spontaneous Processes and Entropy A spontaneous process is a physical or chemical change that occurs by itself. The relative magnitudes of the energy changes.
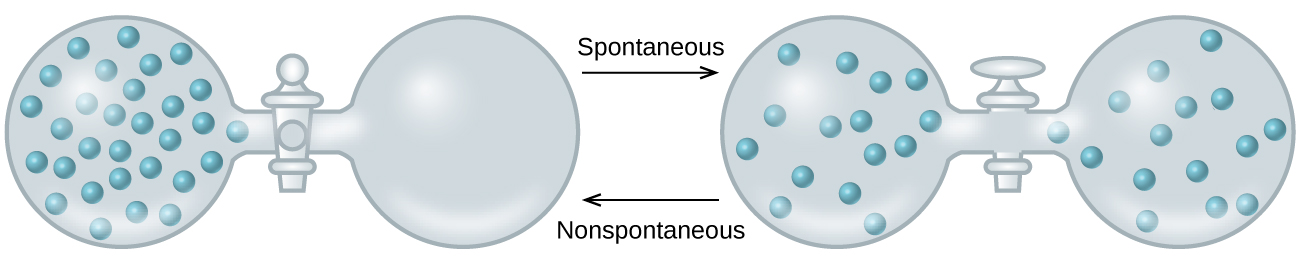
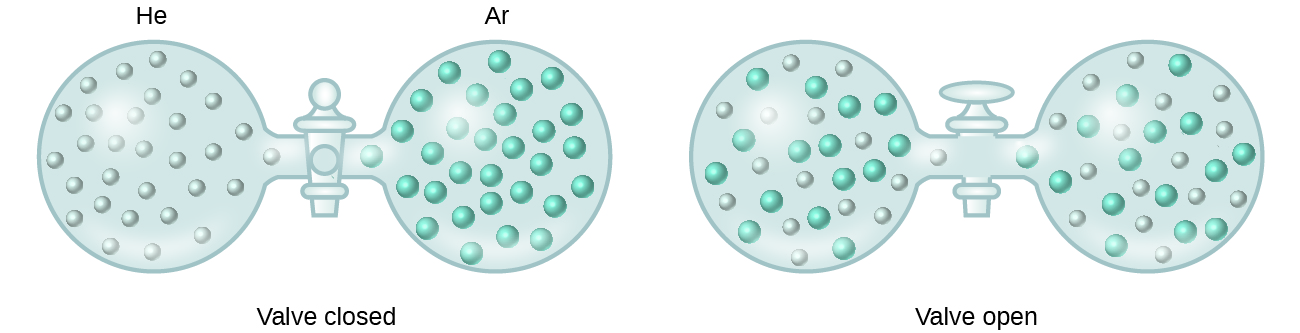
A spontaneous process occurs without the need for a continual input of energy from some external source while a nonspontaneous process requires such. The formation of this solution clearly involves an increase in disorder since the helium and argon atoms occupy a volume twice as large as that which each occupied before mixing. We can say that making a mess is definitely a more spontaneous process.
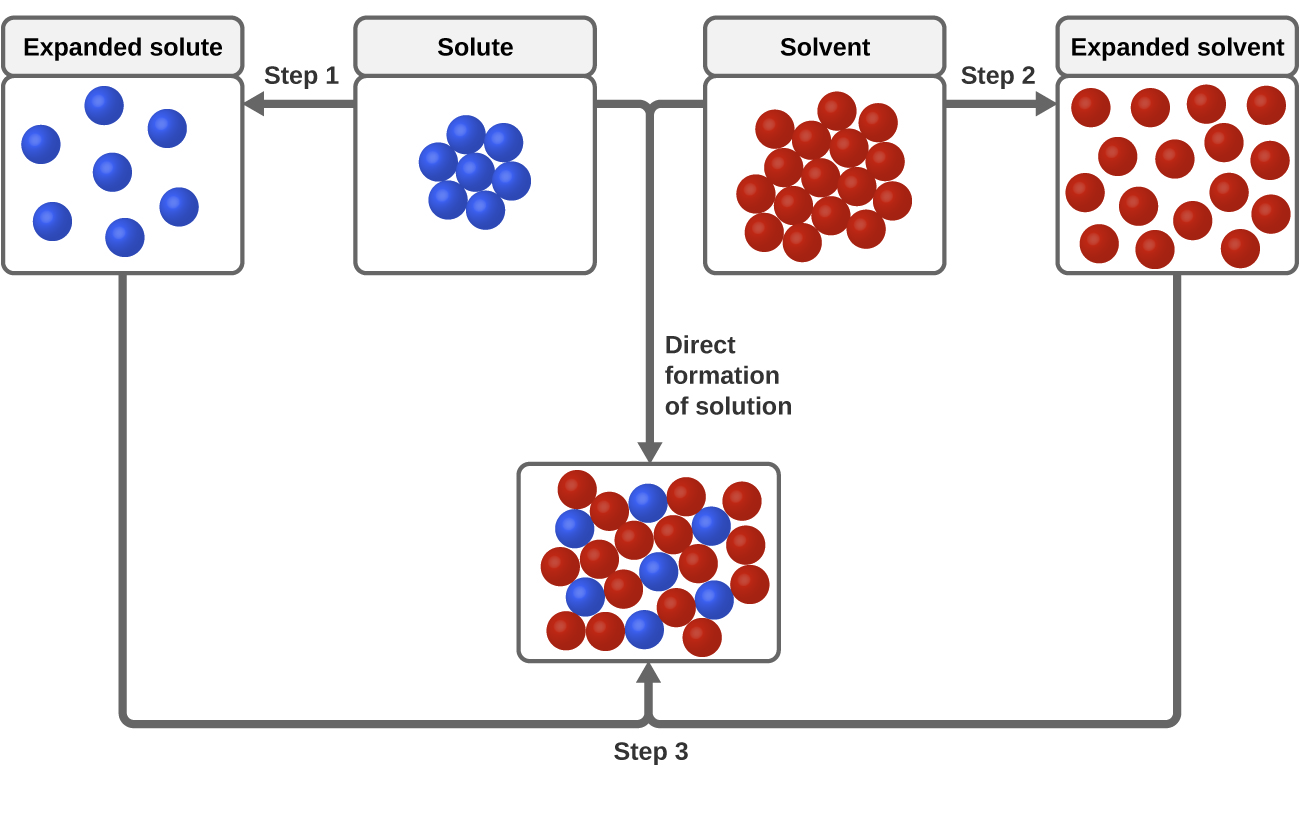
When containers of helium and argon are connected the gases spontaneously mix due to diffusion and form a solution. As illustrated in Figure 3 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation. What is a solute and what is a solvent.
The second law of thermodynamics explains why spontaneous processes have a direction. What is a solute and what is a solvent. The delta H in this process is negative which means that the adsorption is a spontaneous process and it is also an exothermic process.
Ethanol is actually miscible in water which means that the two liquids can be mixed in. Why Adsorption Is Spontaneous Process. 2 Dissolution of Ammonium Chloride is spontaneous because in the solid the ions are fixed but when they go into the aqueous solution they are free to move about.
Heat flows from a hot object to a cold one. What are some traits of a solution. This leads to a bond formation between atom of one amino acid and N atom of other amino acid.
Sometimes we stir a mixture to speed up the dissolution process but this is not necessary. Spontaneous Processes A process that is spontaneous in one direction is not spontaneous in the opposite direction. In any spontaneous process the entropy of the universe increases.
A nonspontaneous process on the other hand will not take place unless it is driven by the continual input of energy from an external source. According to the Second Law of Thermodynamics a reaction is spontaneous if the overall entropy or disorder increases. The direction of a spontaneous process can depend on temperature.
A process that is spontaneous in one direction under a particular set of conditions is nonspontaneous in the reverse direction. A spontaneous reaction may involve an increase or decrease in enthalpy it may involve an increase or decrease in entropy but it will always involve a decrease in free energy that is a negative ΔG. No solution of solute-solute or solvent-solvent forces greater than solvent-solute forces.
Mg OH₂ s Mg² aq 2 OH aq Adding Mg OH₂ will. Two criteria that favor the spontaneous formation of a solution. Adsorption is a spontaneous process because there is a force of attraction existing between the adsorbate and adsorbent which releases heat energy.
Spontaneous solution formation - usually exothermic. The process is spontaneous because it is accompanied by increase of randomness. This is a dehydrationelimination step.
Outside intervention is something that changes the process after it has started. A rock at the top of a hill rolls down. In this process OH which is attached to carbon and H that is attached to the nitrogen atom are eliminated out as H 2 O molecule.
The relative magnitudes of the energy changes. S univ S sys S Surr the change in entropy of the universe is the sum of the change in entropy of the system and the change in entropy of the surroundings. As illustrated in Figure 3 the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions endothermic processes and released when solute-solvent attractions are established an exothermic process referred to as solvation.
Although much of the explanation for why certain substances mix and form solutions and why others do not is beyond the scope of this class we can get a glimpse at why solutions form by taking a look at the process by which ethanol C 2 H 5 OH dissolves in water. Nitrogen will donate its lone pair meaning there will be an attack of nucleophile on the electrophile. So we know that this process of fossil if its performing violators for membranes is spontaneous because its energetically favourable for the 100 filic regions to be on the outside and the hydrophobic regions to be on the inside.
Spontaneous process formation of a solution a process that occurs under specified conditions without the requirement of energy from some external source. A spontaneous process is one that occurs naturally under certain conditions. Why Do Solutions Form.
Systems undergoing a spontaneous process may or may not experience a gain or loss of energy but they will experience a change in the way matter andor energy is distributed within the system. Reversible and Irreversible Processes A reversible process is one that can go back and forth.

Spontaneous Processes Ck 12 Foundation

Designing Learning For A 21st Century Workforce Learning Framework Learning Strategies Learning Theory

What Happens To Energy During The Formation Of A Solution Lisbdnet Com

Thermochemistry Mcqs Pin 4 In 2021 Chemistry Paper Chemistry State Function

Click To Download Redox Rules Posters For Vce Chemistry Teaching Chemistry High School Science Ap Chemistry

Spontaneous Processes Ck 12 Foundation

What Happens To Energy During The Formation Of A Solution Lisbdnet Com

13 2 Types Of Solutions And Solubility Chemistry Libretexts

Thermochemistry Mcqs Pin 4 In 2021 Chemistry Paper Chemistry State Function
11 1 The Dissolution Process Chemistry

Graphic Summary Of Soil Formation Through Weathering Site Has Comprehensive Upper Level Content For Soil Geology Science Facts Earth Science Teaching Science

Crystals Formed In The Gelatin Matrix Sem Images Of Regular Dendrites Optical Microscope Image Of Dense Branching Morphology Credit Goes To Ryuta Ise Yuya
11 1 The Dissolution Process Chemistry

Spontaneous Processes Ck 12 Foundation
18 2 Spontaneous And Nonspontaneous Processes Chemistry Libretexts

Spontaneous Formation And Base Pairing Of Plausible Prebiotic Nucleotides In Water Nature Communications Molecular Biology Spontaneous Molecular

What Happens To Energy During The Formation Of A Solution Lisbdnet Com